This website is intended for Health Care Professionals in Croatia.
Za Informacije o lijeku kliknite ovdje
Informacije o prijavljivanju nuspojava nalaze se na dnu stranice
Za Informacije o lijeku kliknite ovdje.
Informacije o prijavljivanju nuspojava nalaze se na bottom dnu stranice
FORXIGA (dapagliflozin) i kronična bubrežna bolest
Forxiga (dapagliflozin) is an SGLT2i recommended by NICE for the treatment of CKD1

Dapagliflozin remains the only SGLT2i to reduce the risk of all cause mortality in patients with CKD on top of SoC vs placebo2
The approval of dapagliflozin for the treatment of chronic kidney disease was a significant advancement in CKD in more than 20 years.3,4 Dapagliflozin provides a once daily oral treatment for patients with CKD on top of standard of care therapy (angiotensin-converting enzyme inhibitor [ACEi] or angiotensin receptor blocker [ARB])2
The unmet need in CKD
CKD is a progressive disease that significantly impacts quality of life and increases the risk of:5
Poor cardiovascular (CV) and renal outcomes
Premature death
Heart Failure
Early intervention is important
CKD leads to a gradual loss of kidney function, and can influence the progression of type 2 diabetes (T2D) and heart failure (HF), worsening overall prognosis5-9
On top of SoC vs placebo, in people with CKD with and without T2D, FORXIGA reduces the composite risk of:2*
- Declining kidney function (≥50% sustained decline in eGFR)
- Renal or CV death
- End-stage kidney disease (ESKD)
- Declining kidney function (≥50% sustained decline in eGFR)
- Renal or CV death
- End-stage kidney disease (ESKD)
CKD Prevalence
7.2 million
people were living with CKD in the United Kingdom (UK) in 202210
~45,000
premature deaths occur every year in individuals with CKD in the UK5
Impact of CKD on the National Health Service (NHS)
£6.4 billion
is the estimated cost of kidney disease to the NHS in 202310
Almost 50%
of all CKD-related hospitalisations are accounted for by frequently-hospitalised non-dialysis patients, of which most cases are attributed to HF and hyperkalemia11
Unplanned (emergency) hospital admissions are common in people with CKD, and are more likely as CKD worsens.10
39 more
unplanned hospital admissions occur annually per 100 patients with Stage 4 vs. Stage 3 CKD12
12 more
patients die annually per 100 patients with Stage 4 vs. Stage 3 CKD12
FORXIGA (dapagliflozin) CKD trials
DAPA-CKD was a landmark study that investigated the use of dapagliflozin in the treatment of chronic kidney disease (CKD) patients, with or without type 2 diabetes (T2D)2
DAPA-CKD was a randomised, double-blind, placebo-controlled, multicentre clinical trial in patients with CKD, to determine the long-term efficacy and safety of the SGLT-2i FORXIGA (dapagliflozin) in patients, with and without T2D, on top of standard care2,10

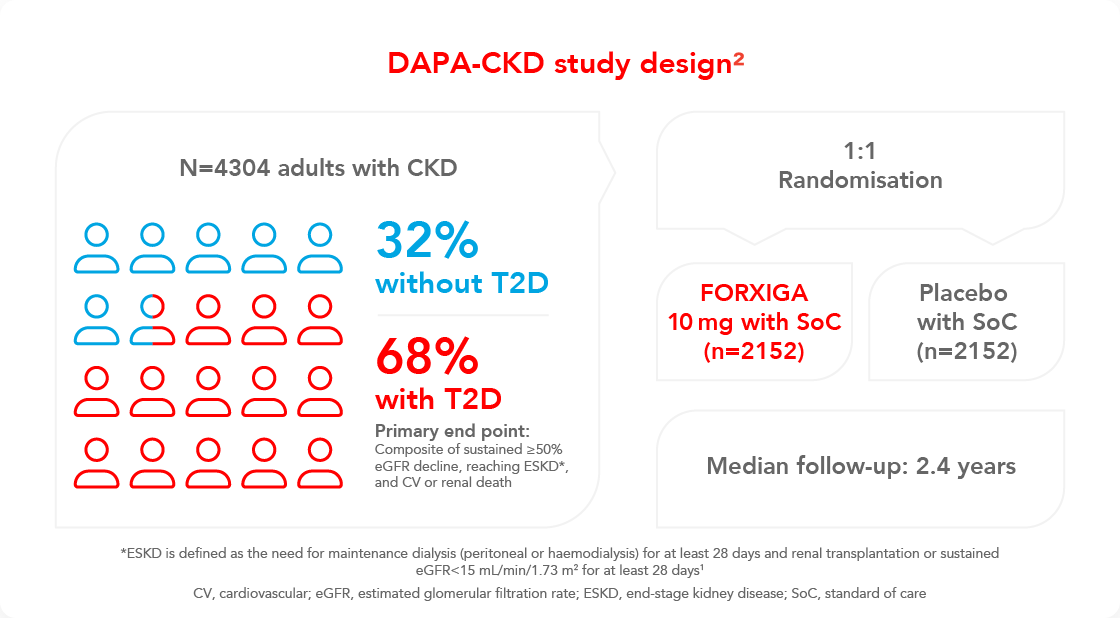
DAPA-CKD inclusion criteria:2
- Estimated glomerular filtration rate (eGFR) range: 25–75 mL/min/1.73m²
- Urine albumin creatinine ratio range:
22.6–565 mg/mmol - Participants were required to be receiving a stable and maximum-tolerated labelled dose of an angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) for at least 4 weeks prior to screening
Key exclusion criteria:2*
- Patients with polycystic kidney disease, lupus nephritis, or antineutrophil cytoplasmic antibody-associated vasculitis
- Recent treatment with, or unacceptable side effects associated with a SGLT-2i within 8 weeks prior to enrolment2
- Patients treated with cytotoxic or immunosuppressive therapy for primary or secondary kidney disease 6 months prior to study enrolment
- Patients with a history of organ (including kidney) transplantation
- Patients with type 1 diabetes
*This list is not exhaustive, please refer to the supplementary appendix for the full list of key exclusion criteria²
FORXIGA (dapagliflozin) and CKD: efficacy
FORXIGA (dapagliflozin) in patients with CKD, with or without type 2 diabetes (T2D):
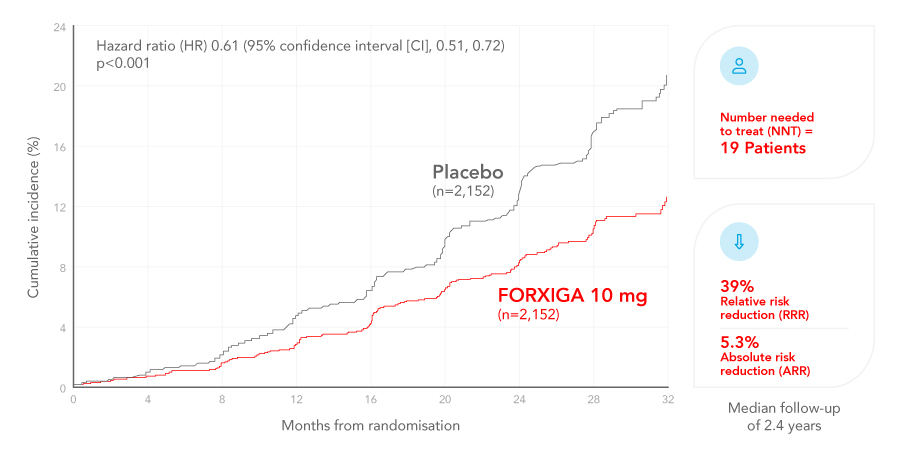
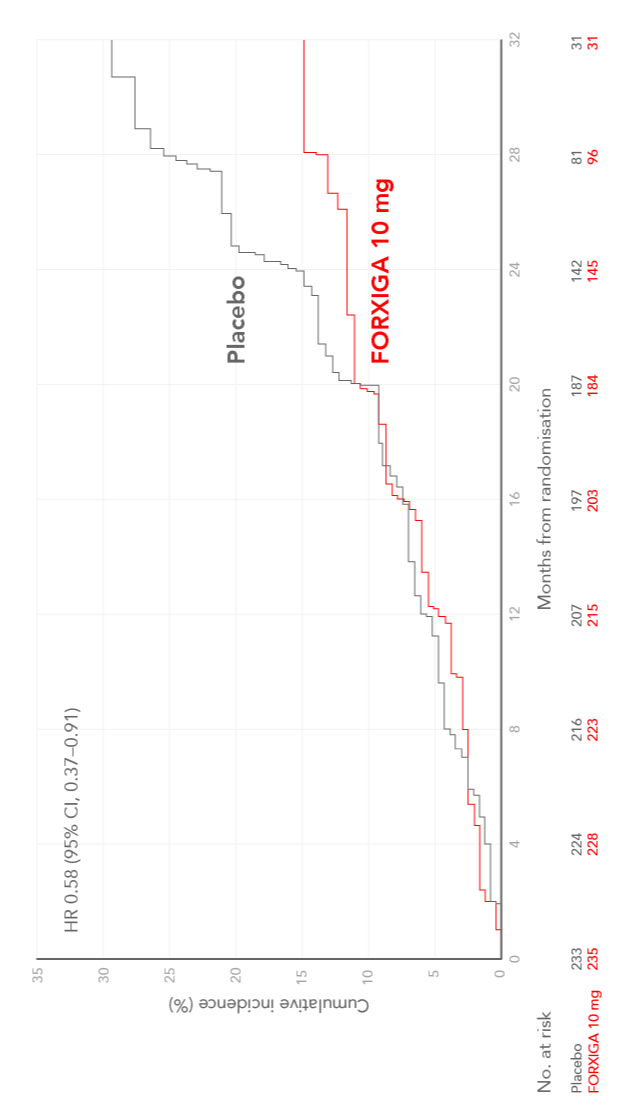
FORXIGA (dapagliflozin) reduces the risk of the composite of declining kidney function, ESKD and renal or CV death on top of standard of care compared with placebo2,13
Cumulative incidence of sustained decline in the eGFR of at least 50%, ESKD, or death from CV or renal causes in patients with CKD, with or without type 2 diabetes (T2D)2,14
Adapted from Heerspink et al. 2020
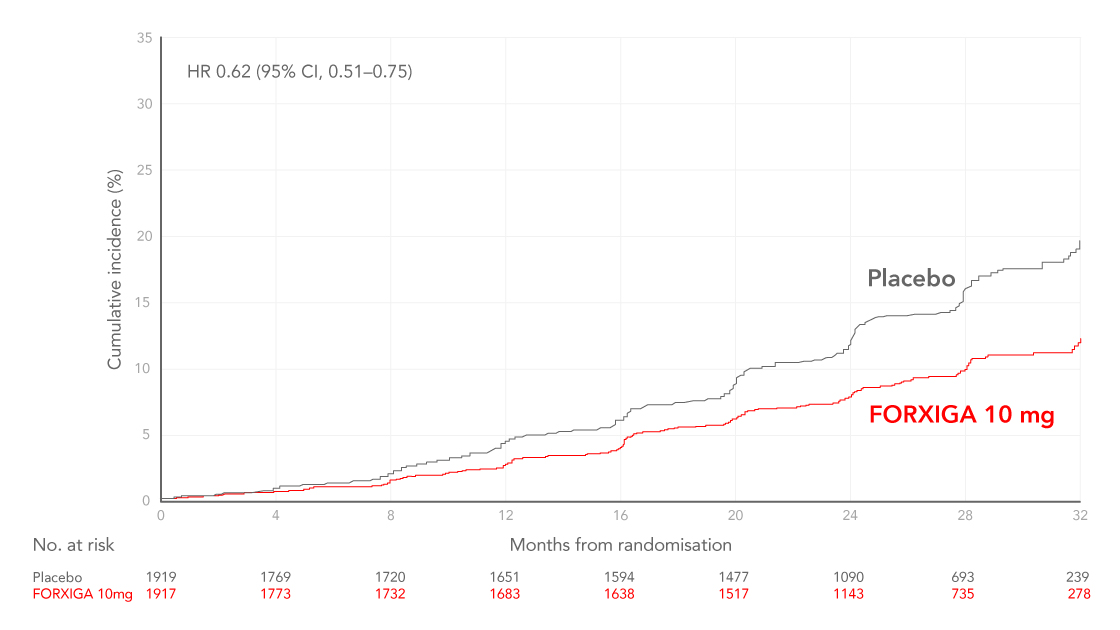
FORXIGA (dapagliflozin) in patients with CKD, with or without a history of HF:
Prespecified analysis: There were fewer events of declining kidney function, ESKD, and renal or CV death with FORXIGA 10 mg compared with placebo2
Cumulative incidence of the composite of a sustained decline in eGFR of at least 50%, ESKD, or death from renal causes in patients with or without type 2 diabetes (T2D), shown first in patients with no history of HF and then shown in patients with HF15
Adapted from McMurray et al. 2021
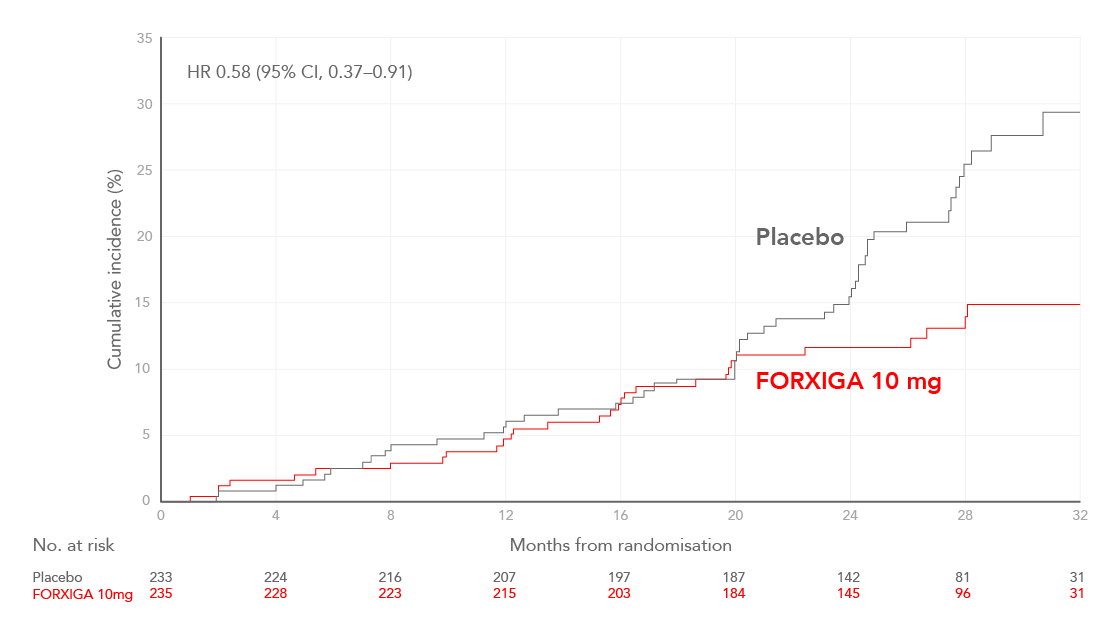
Cumulative incidence of the composite of a sustained decline in eGFR of at least 50%, ESKD, or death from renal causes in patients with or without type 2 diabetes (T2D), shown first in patients with HF and then shown in patients without HF15
Adapted from Heerspink et al. 2020
Key secondary efficacy endpoints:
31%RRR
in all-cause mortality vs. placebo¹
2.1%ARR
(4.7% vs. 6.8%) HR 0.69; 95% CI, 0.53, 0.88; p=0.004.
29%RRR
in the composite of CV death or hospitalisation for heart failure (hHF) vs. placebo, driven by hHF¹
1.8%ARR
(4.6% vs. 6.4%) HR 0.71; 95% CI, 0.55, 0.92; p=0.009.
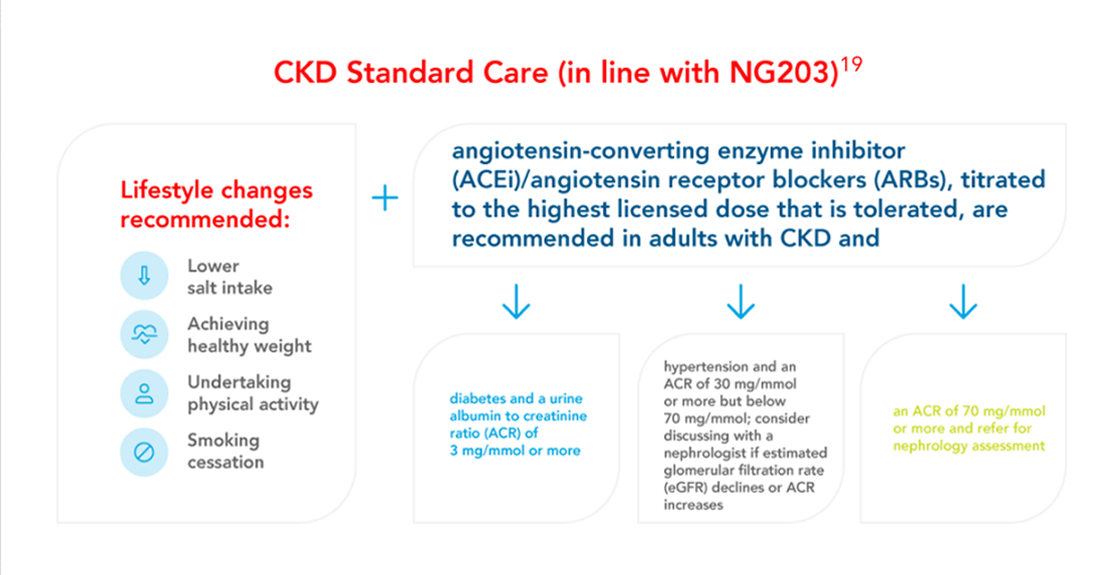
FORXIGA (dapagliflozin) in CKD treatment guidelines
FORXIGA is recommended for the management of chronic kidney disease (CKD) by a number of major guidelines and treatment recommendations
National guidelines (UKKA, NICE and ABCD) recommend FORXIGA (dapagliflozin) for the management of CKD in patients with or without type 2 diabetes (T2D)1,16-20
Dose adjustments are not required based on renal function
Forxiga (dapagliflozin) offers simple, once daily dosing11 across the range of eGFR.
In patients with severe hepatic impairment, a starting dose of 5 mg is recommended. If well tolerated, the dose may be increased to 10 mg.
There is limited experience with initiating FORXIGA (dapagliflozin) treatment in patients with eGFR <25 mL/min/1.73m2, and no experience in patients with eGFR <15 mL/min/1.73m2. Therefore, it is not recommended to initiate treatment in patients with eGFR <15 mL/min/1.73m2
Sign up and stay up to date
Stay informed on the latest product updates and news.
HCP and Patient resources
Support your patients and expand your knowledge with essential resources.
CKD increases CV risk
Learn about the role of dapagliflozin in managing people with HF.
Contact Us
If you have any questions about FORXIGA or would like to speak to an AstraZeneca account manager, please contact us
Skračenice:
ABCD, Association of British Clinical Diabetologists; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARR, absolute risk reduction; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; HF, heart failure; HR, hazard ratio; NICE, National Institute for Health and Care Excellence; NNT, number needed to treat; RRR, relative risk reduction; SGLT2i, Sodium/glucose cotransporter-2 inhibitors; SoC, standard of care; T2D, type 2 diabetes; UACR, urine albumin-creatinine ratio; UK, United Kingdom; UKKA, UK kidney association.
Reference:
- NICE Technology Appraisal Guidance: Dapagliflozin for treating Chronic Kidney Disease [TA775]. March 2022. Available from: https://www.nice.org.uk/guidance/ta775. Last accessed: November 2023. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this material.
- Heerspink HJL et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436–1446.
- Breyer MD et al. Nat Rev Drug Discov. 2016;15(8):568-588.
- Tuttle KR. Lancet Diabetes Endocrinol. 2021;9(1):3-5.
- Kerr M. Chronic Kidney Disease in England. the Human and Financial Cost. NHS. 2012.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(1):1–150.
- Birkeland KI et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: A large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607-1618.
- Rawshani A. et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379(7):633-644.
- Alicic RZ, et al. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045.
- Kidney disease: A UK public health emergency (2023) Kidney Research UK. Available at: https://www.kidneyresearchuk.org/about-us/influencing-change/health-economics-report/. Last accessed: November 2023.
- Ronksley et al. Potentially Preventable Hospitalization among Patients with CKD and High Inpatient Use. Clin J Am Soc Nephrol. 2016 Nov 7;11(11):2022-2031
- National Chronic Kidney Disease Audit, National Report: Part 2, 2017. Available at: http://allcatsrgrey.org.uk/wp/wpfb-file/final-ckd-audit-report-14-dec-2017-pdf/. Last accessed: November 2023
- Cjelokupni opis svojstava lijeka FORXIGA 10 mg.
- Wheeler DC, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22-31.
- McMurray JJV, et al. Effects of Dapagliflozin in Patients With Kidney Disease, With and Without Heart Failure [published correction appears in JACC Heart Fail. 2022 Jun;10(6):446-447]. JACC Heart Fail. 2021;9(11):807-820.
- UK Kidney Association Clinical Practice Guideline: Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibition in Adults with Kidney Disease. Working Group co-chairs: Assoc. Prof William G. Herrington & Dr Andrew H. Frankel. Final Version: 13 April 2023. Available at: https://guidelines.ukkidney.org/2023-update/
- NICE Technology Appraisal Guidance: TA679 (24th February 2021). Dapagliflozin for treating heart failure with reduced ejection fraction. Publication date: February 2021. Available from: https://www.nice.org.uk/guidance/ta679. Last accessed: November 2023. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this material.
- NICE guideline NG28: Type 2 diabetes in adults: management. Publication date 15th February 2022. Available from: https://www.nice.org.uk/guidance/ng28. Last accessed: November 2023. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this material.
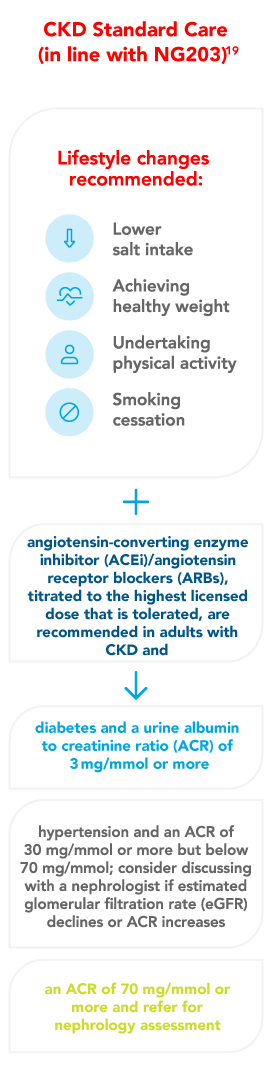
- Chronic kidney disease: assessment and management NICE guideline [NG203]. Available from: https://www.nice.org.uk/guidance/ng203. Last accessed: November 2023. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this material.
- Basu A et al. Cardiovascular impact of new drugs (GLP-1 and gliflozins): the ABCD position statement BJD. 2021; 21(1):132-148.
kod stranice